ASA Activist Newsletter - November 2017
In the November 2017 Issue:
- Rallies Call for Cannabis Solution to Opioid Crisis
- ASA Files Freedom of Information Requests on Opioid Commission
- ASA Chief Scientist Co-Authors JAMA Study on CBD Product Labeling
- Cannabis Expert Dr. Ethan Russo Joins Center of Excellence
- PFC Trainings, Outreach and Events Span the US
- Activist Profile: Justin Arriola, Utah
- ACTION ALERT: Support the CJS Cannabis Amendment Today!
________________________
Rallies Call for Cannabis Solution to Opioid Crisis
![]() The role medical cannabis can play in addressing the opioid crisis was the focus of rallies advocates held on November 1. In Washington, D.C., Americans for Safe Access, the US Pain Foundation, and approximately 150 advocates held a press conference and rally with Congressman Earl Blumenauer (D, OR-03; pictured) to encourage policymakers to consider medical cannabis as an option for pain.
The role medical cannabis can play in addressing the opioid crisis was the focus of rallies advocates held on November 1. In Washington, D.C., Americans for Safe Access, the US Pain Foundation, and approximately 150 advocates held a press conference and rally with Congressman Earl Blumenauer (D, OR-03; pictured) to encourage policymakers to consider medical cannabis as an option for pain.
Following the D.C. action, advocates delivered a letter to the leadership of the Senate and House Committees on Appropriations urging them to include the medical cannabis amendment in the FY18 appropriations package. The amendment prevents the Department of Justice from using any funds to interfere with state medical cannabis programs, including federal prosecution of state-compliant individuals. The letter was signed by the National Multiple Sclerosis Society, Michael J. Fox Foundation, US Pain Foundation, Epilepsy Foundation, Tourette Association of America, National Women’s Health Network, Realm of Caring, and Americans for Safe Access.
“The opioid crisis is a national emergency, killing 147 people a day. We must do more to help the families and communities torn apart by addiction,” said Rep. Earl Blumenauer (OR-03). “At the very least, the federal government should stay out of the way as states allow access to safer alternatives to opioids like medical cannabis.”
The medical cannabis amendment has been included in the federal budget each year since 2015, but this year the House Committee on Rules blocked a floor vote. The Senate version of the amendment (introduced by Senator Leahy) was passed in July in the Senate FY2018 Commerce, Justice, Science and Related Agencies Appropriations bill.
The November rallies come on the heels of the Administration’s formal announcement last week declaring the opioid crisis a public health emergency. That declaration does not include provisions for additional funds states need to combat the crisis, nor does it acknowledge the potential role of medical cannabis in fighting the epidemic.
A study in the Journal of the American Medical Association found that when states implemented medical cannabis programs unintentional opioid overdose deaths dropped by 25%. That study also showed a 13% decrease in hospitalizations from opioid-related causes. . In a survey of nearly 3,000 pain patients, 93% preferred medical cannabis over opioid therapies for pain management.
Many of the states with the highest opioid overdose rates, such as West Virginia, New Hampshire, and Ohio, have medical cannabis programs that allow it to be used to treat chronic pain.
“We call on the Administration and Congress to take measures to ensure medical cannabis states have the protections and federal support they need,” said ASA Executive Director Steph Sherer. “Medical cannabis is a safe, effective treatment for pain that can decrease opioid addiction and overdose.”
More Information:
ASA Report: Medical Cannabis Access for Pain Treatment, A Viable Strategy to Address the Opioid Crisis
________________________
ASA Files Freedom of Information Requests on Opioid Commission
![]() Following the release of the White House opioid report, Americans for Safe Access has asked for information about what was discussed about medical cannabis.
Following the release of the White House opioid report, Americans for Safe Access has asked for information about what was discussed about medical cannabis.
ASA’s two requests under the Freedom of Information Act (FOIA), filed November 9, seek disclosure of how the President’s Commission on Combating Drug Addiction and the Opioid Crisis draft final report came to include only a single cannabis study. That study only used survey data obtained before medical cannabis programs were operating in the United States. The commission’s report omits all the available clinical research data on the safety and effectiveness of cannabis for treating chronic pain.
The FOIA requests were sent to the White House Office of National Drug Control Policy (ONDCP) and the National Institute of Drug Abuse.
“The President’s Opioid Commission presented grossly misleading information in its final report, excluding proven strategies such as medical cannabis that can reduce opioid deaths,” said Steph Sherer, ASA Executive Director. “They did not use the best available science. Americans concerned about opioid addiction need to know why the Commission used one marginal study to dismiss cannabis as a therapeutic alternative.”
The Commission was formed just months after the National Academies of Sciences, Engineering, and Medicine released “The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research (2017),” which compiled research from over 10,000 studies on cannabis and its components. The report found that “in adults with chronic pain, patients who were treated with cannabis or cannabinoids are more likely to experience a clinically significant reduction in pain symptoms” and “there is substantial evidence that cannabis is an effective treatment for chronic pain in adults.”
In the introductory letter to the report, Governor Chris Christie (R-NJ), the chair of the committee, writes:
“The Commission acknowledges that there is an active movement to promote the use of marijuana as an alternative medication for chronic pain and as a treatment for opioid addiction. Recent research out of the NIH’s National Institute on Drug Abuse found that marijuana use led to a 2-1⁄2 times greater chance that the marijuana user would become an opioid user and abuser. The Commission found this very disturbing.”
Thirty states in the US have passed medical cannabis laws and another 16 have passed more limited laws around Cannabidiol (CBD). Research shows that opioid deaths decreased in states that adopted medical cannabis laws by an average of 25%. A separate NIH study found that medical cannabis legalization was associated with a 23% reduction in hospitalizations related to opioid dependence or abuse and a 13% reduction in opioid pain relief overdose.
Those findings reflect the degree to which patients who rely on opioid drugs for pain relief are able to substitute medical cannabis. Researchers from the University of California, Berkeley and Kent State University found in a study of 2,897 pain patients that “97 percent of the sample 'strongly agreed/agreed' that they are able to decrease the amount of opioids they consume when they also use cannabis.”
“We want to know why and how the peculiar study Gov. Christie cited came about,” said Sherer. “The timing is curious, as NIDA appears to have rushed it out in order to give the Commission a reason to ignore the compelling scientific evidence and the thousands of inquiries the Commission received during the public comment period about using medical cannabis to help with the crisis.”
NIH and ONDCP have 20 Federal business days to deliver, ask for an extension, or deny the requested documents to Americans for Safe Access.
More Information:
FOIA Requests to NIDA and ONDCP
Opioid Commission Report
ASA Report: Medical Cannabis Access for Pain Treatment
________________________
ASA Chief Scientist Co-Authors JAMA Study on CBD Product Labeling
 Nearly 70 percent of all cannabidiol (CBD) products sold online are either over or under labeled, according to a new study co-authored by Jahan Marcu, Ph.D, ASA Chief Science Officer and Director of Patient Focused Certification (PFC). The study, which was published in the prestigious Journal of the American Medical Association (JAMA), analyzed 84 CBD products available online from 31 companies.
Nearly 70 percent of all cannabidiol (CBD) products sold online are either over or under labeled, according to a new study co-authored by Jahan Marcu, Ph.D, ASA Chief Science Officer and Director of Patient Focused Certification (PFC). The study, which was published in the prestigious Journal of the American Medical Association (JAMA), analyzed 84 CBD products available online from 31 companies.
“This is a wake-up call for the CBD industry to standardize their products,” said Dr. Marcu, “CBD product manufacturers need to adopt best practices and accept guidance from AHPA and other groups to improve consistency and safety for consumers. Reaching compliance with existing standards for cannabis-products could help address this issue.”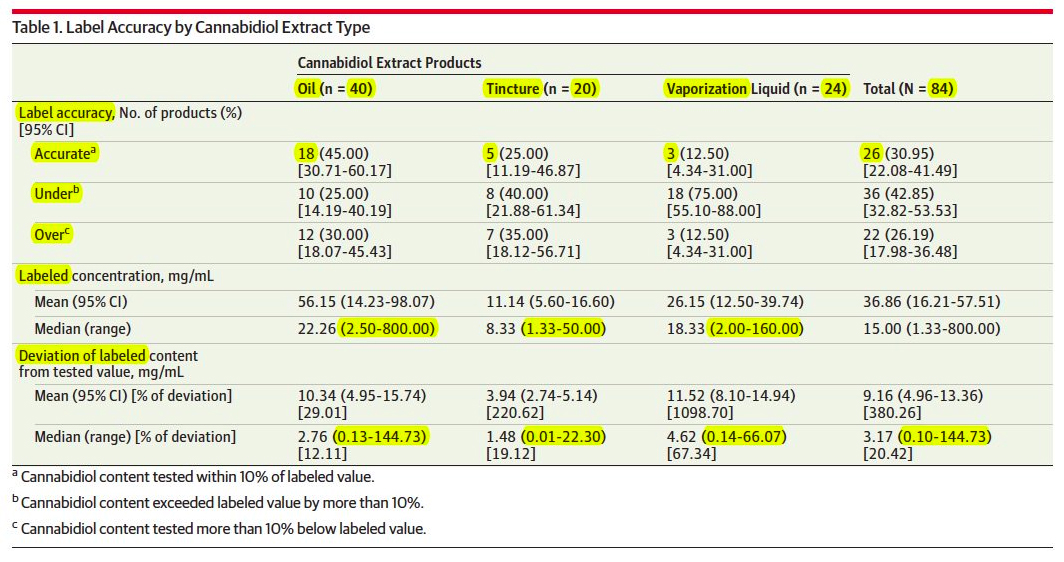
Analysis from jointly PFC certified & ISO17025 accredited laboratories showed 43 percent of products contained more CBD than indicated on the label, 26 percent contained less, and 31 percent were accurately labeled within 10 percent. This degree of mislabeling creates challenges for establishing and maintaining safe and effective dosages, particularly since these CBD formulations are often used to treat children with epilepsy.
Interest in the medicinal use of CBD, a chemical in the cannabis plant, has increased with accumulating evidence that it has therapeutic benefits without intoxication or risk of abuse. Recent research supports use of CBD for children with serious seizure disorders, and patients in states where CBD products are legally available report it can be effective for a variety of health conditions.
The market for CBD products may grow to more than $2 billion in consumer sales within the next three years, according to industry analysts. Yet little has been done to ensure regulation and oversight of the manufacture and sale of products containing CBD.
Like cannabis, CBD is currently classified by the DEA as a Schedule I controlled substance with no current accepted medical use, despite being available for medicinal use in many states. Many online retailers sell CBD products, though the FDA has issued warnings about making medical claims about those products.
Dr. Marcu is one of six co-authors, including researchers from Johns Hopkins and the University of Pennsylvania. Dr. Marcu has more than 10 years of experience in cannabis pharmacology and chemistry, and is an expert on medical cannabis standards. He is co-author of The American Herbal Pharmacopoeia Cannabis Inflorescence Standards of Identity, Analysis, and Quality Control and other peer-reviewed reports published by Americans for Safe Access.
More Information:
Labeling Accuracy of Cannabidiol Extracts Sold Online
________________________
Cannabis Expert Dr. Ethan Russo Joins Center of Excellence
Last month, the International Cannabis and Cannabinoids Institute (ICCI) announced that renowned medical cannabis researcher Ethan Russo, MD has been named the institute’s new Director of Research and Development. ICCI, founded in the Czech Republic in partnership with Americans for Safe Access, is the first international Center of Excellence for the advancement of cannabis and cannabinoid treatments. ICCI identifies, coordinates, and supports global research priorities for the advancement of cannabis and cannabinoid treatments through a multidiscipline evidence-based approach that incorporates innovative tools and approaches.
“We are honored to have Ethan Russo join the team at ICCI,” said Steph Sherer, ASA Executive Director.” His expertise will be key to advancing many of the important projects underway, including our Global Scientific-Based Biomedical, Life Science, and Policy Research Priority Mapping Project, International Call for Standardization of Data in Cannabis Research and Adverse Event Reporting and clinical trials such as our Opioids and Cannabinoids in the treatment of pain study.”
Ethan Russo, MD, is a board-certified pediatric and adult neurologist, psychopharmacology researcher, former Senior Medical Advisor to GW Pharmaceuticals, and Medical Director of PHYTECS. He served as study physician to GW Pharmaceuticals for numerous Phase I-III clinical trials of Sativex®, and early stage investigations of Epidiolex®. He has held faculty appointments in Pharmaceutical Sciences at the University of Montana, in Medicine at the University of Washington, and as visiting professor at the Chinese Academy of Sciences. He is a Past-President of the International Cannabinoid Research Society, and former Chairman of the International Association for Cannabinoid Medicines.
“I am delighted to join the ICCI team and collaborate on bringing cannabis-based medicines and industrial hemp products back into the mainstream for the patients and public that will benefit from their use,” Russo remarked. “The research capabilities of ICCI personnel and its development partners internationally will synergize advancements in healthcare that portend to improve quality of life for people across the world.”
As an international collaborative project of nonprofit, for-profit, and government institutions, ICCI has developed strategic project partnership alliances with universities, biotech companies, and research institutions to create a patient-centered international research and educational hub. ICCI employs a cross-disciplinary approach that captures developments across the fields of biomedical research, social science, life sciences, and policy research.
“It’s a great honor to welcome leading expert and medical cannabis professional Ethan Russo to ICCI,” says Pavel Kubu, ICCI CEO. “ICCI’s research and development activities are rapidly growing. Dr. Russo’s experience is an exciting addition to our team.”
Americans for Safe Access co-founded ICCI in 2016 to help remove barriers to access for patients globally.
For more information:
www.ICCI.science
________________________
PFC Trainings, Outreach and Events Span the US
 ASA’s Patient Focused Certification continues its busy schedule of trainings and outreach. In the last week of October, PFC was in Nashville, Tennessee working with advocates and appearing before the state task force on medical cannabis. Officials said they were looking to PFC for how to implement standards in the state. PFC will be back in Tennessee in early 2018 training regulators, stakeholders and patients as part of a tour that will include Arkansas and Louisiana.
ASA’s Patient Focused Certification continues its busy schedule of trainings and outreach. In the last week of October, PFC was in Nashville, Tennessee working with advocates and appearing before the state task force on medical cannabis. Officials said they were looking to PFC for how to implement standards in the state. PFC will be back in Tennessee in early 2018 training regulators, stakeholders and patients as part of a tour that will include Arkansas and Louisiana.
The work in Tennessee was followed by trainings in Maryland at the beginning of November, where license applicants are preparing for mandatory state inspections.
ASA’s Patient Focused Certification team was in Las Vegas at the MJ Biz Conference from November 15-17. PFC talked to industry professionals about training, education and business certification, as well as ASA’s opioid campaign, End Pain Not Lives.
Following the MJ Biz conference, PFC will be back in Maryland until December 8, helping prepare medical cannabis businesses for state inspections. Submissions for Stage 2 licenses require on-site inspections by the Maryland Medical Cannabis Commission at a mutually agreed time prior to December 8. Applicants who are interested in PFC’s pre-licensing inspection assistance can make appointments via the PFC website at patientfocusedcertification.org.
In December, ASA Executive Director Steph Sherer and PFC Director Jahan Marcu, PhD will be attending the annual Emerald Cup in Santa Rosa, California. On Saturday, December 9, Dr. Marcu will give an invited talk on the pros and cons of extraction methods, considering how to ensure safe methods and products. On Sunday, December 10, Steph Sherer will give a talk on the future of medical cannabis. She will also be honored with the Emerald Cup’s most prestigious individual award.
Check the PFC website for all the upcoming opportunities, as well as online webinars and Facebook live events such as Cannabis Science Corner with ASA Chief Science Officer and PFC Director Jahan Marcu, PhD. If you have a question for Dr. Marcu, you can submit it on the PFC facebook page.
________________________
Activist Profile: Justin Arriola, Utah
Navy veteran Justin Arriola, a former petty officer 3rd class who served in the Gulf, knows cannabis can help treat the effects of the herniated disc he suffered in the line of duty, but he doesn’t consider himself a patient. It was his mother’s breast cancer diagnosis that made him a public advocate.
Justin’s mother still speaks about her cannabis in whispers and doesn’t want her sisters to know, so Justin speaks for not just her but the other members of his family who have had to live with cancer. He also sees what a difference safe access to medical cannabis could make for other veterans, as well as the many Utahans who are confronting opiate addiction.
But legal consequences and stigma are still substantial in Utah, so speaking out has not been without risk. Justin had to think long and hard about the effects advocacy might have on his engineering business. He’d learned quite a lot about medical cannabis and was already applying his technical expertise to helping patients by consulting on setting up cultivation, working through an underground network of doctors and nurses facilitating medicine to individuals.
He was also chasing engineering jobs all over the country, which took him to Washington State and other places with robust medical cannabis programs. He used his industrial skills to help providers scale up from clandestine, hand-watered cultivation to automated 20,000-plant facilities with remote monitoring. He took classes and got certified in Colorado on extraction techniques.
As Justin learned the science, he met more patients and became privy to their stories. He saw the success people in other states. Seeing the people fighting finally have access and get the life they’d been fighting for helped him understand the need to speak out.
“When you’re really helping patients, particularly families with kids, it makes a difference,” he says. “To see them have a conversation with their kid when they couldn’t before, that’s really gratifying. It solidifies your activism.”
Justin worked on safe access campaigns in the State of Washington, but he assumed he’d never see it happen in Utah. But things have changed.
For the past year, Justin has worked with TRUCE, an advocacy organization in Utah, and is now working with a local veterans group for cannabis. Last May, he received a scholarship to attend ASA’s National Unity Conference, where he connected with other advocates, learned more about cannabis, and lobbied the offices of Senator Orrin Hatch and Representative Jason Chaffetz. That lobbying last spring was part of efforts that helped convince Sen. Hatch to introduce in September the Marijuana Effective Drug Study Act of 2017, or MEDS Act.
“It’s been hot and heavy since we’ve been back,” Justin says. “We’ve got a ballot initiative campaign in Utah, and public opinion has gone through the roof. I think we’re going to get on the ballot and get this passed.”
Utah remains a challenging state for advocacy. The legislature and law enforcement are not friendly, and the church dominates the conversation in most circles. Justin enjoys teaching those in opposition why their thinking is wrong, why medical cannabis is important in a state that ranks 7th in the nation in opiate overdoses.
“I’ve lost friends, both vets and childhood, but I’ve also seen people weaned off heroin who become productive members of society,” says Justin. “That spurs me on to keep fighting.”
Justin worried that becoming a public voice would mean career suicide in a state such as Utah, but the blowback he feared never materialized. In fact, it’s proven to be a net positive. He’s looking forward to being back in D.C. for the ASA Unity Conference next May, where he’s looking to push it national.
“I believe in the plant,” Justin says. “I’ve seen it do wonderful things for both people who are sick with disease and people who are sick with addiction.”
____________________________
ACTION ALERT: Support the CJS Medical Marijuana Amendment Today!
Take action today to continue federal protections for medical cannabis patients and their providers! Congress needs to hear from you why it’s critically important to renew the CJS Medical Marijuana Amendment.
Since 2015, participants in state medical cannabis programs have been protected from federal interference by the CJS Medical Marijuana Amendment, which has to be renewed each year. This year, the Senate passed it, but the House leadership blocked it from coming to a vote. That means Congress will have to “reconcile” the two versions of the FY2018 appropriations package, and the amendment may not survive.
Rhonda Firestack-Harvey, one of the Kettle Falls Five group of medical cannabis patients who were prosecuted and convicted in federal court despite complying with Washington state law, was at the Capitol last week lobbying her elected officials and other members of Congress. Rhonda’s story made a huge impact as policymakers heard firsthand what the CJS medical marijuana amendment means to America’s over two million medical cannabis patients and the businesses that serve them.
The current protections are set to expire on December 8th, so your help is essential to ensure these protections continue in 2018. Contact your Senators and Representative today!
____________________________
Share this page





















